Management of Transfusion-dependent Aplastic Anemia
- sbord1
- Jan 2, 2023
- 4 min read
CASE
Pt was a middle-aged AA woman with PMH of uterine fibroids, adenomyosis, and aplastic anemia who presented to the ED due to a 1-week history of abnormal uterine bleeding.
She normally used 3-5 menstrual pads for her frequent bleeding but has recently soaked through 40-50 heavy flow pads per day.
She went to her PCP who upon seeing her Hgb of 5.9 and platelets of 39 advised her to go to the ED.
Due to prior authorization and insurance issues, she had been without her Provera for her fibroids for 1 week and her cyclosporine for her aplastic anemia for 2 weeks. She was able to receive a limited supply of cyclosporine 2 days before her presentation from her hematologist.
Prior pelvic MRI showed adenomyosis and multiple subserosal, submucosal, and intramural fibroids.
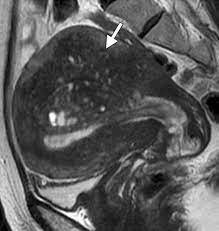
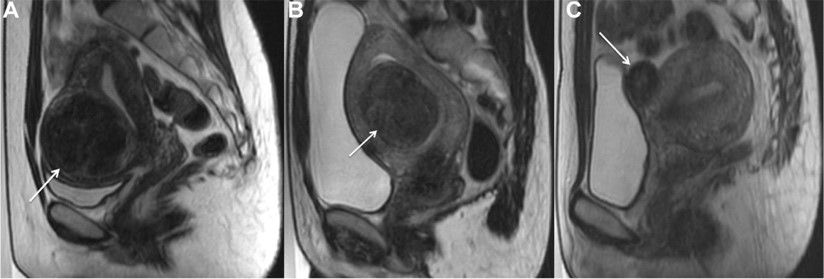
Her BP was 101/74, HR 93, RR 14, and SpO2 99%. On physical exam, she had profound suprapubic tenderness to palpation w/ a boggy, tender uterus palpable below the umbilicus. A large clot covering the cervical os was seen on speculum exam.
During her course in the ED, her Hgb dropped below 5 and her platelets became as low as 20. She was admitted to the gynecology service with hematology following closely. She was crossed and matched for 4 units of pRBCs and received 1 unit. One unit of platelets were also ordered.
She normally takes her cyclosporine and other medications with food, but she hadn’t eaten yet. We deferred administration of her home medications until hematology gave recommendations.
Clinical Question: What is the optimal management of adults who present with transfusion-dependent aplastic anemia?
SUMMARY OF EVIDENCE
Aplastic anemia is “an immune-mediated hematopoietic disorder characterized by pancytopenia and hypocellular bone marrow.”[4]
Transfusions are an important part of the supportive care often necessary.
In those with symptomatic and/or severe anemia, leukoreduced pRBCs should be administered. Removing the leukocytes from the pRBCs decreases the risk of various complications such as transfusion reactions, transmission of viruses, and suboptimal responses to platelet transfusions.[7]
There is not a universally accepted Hgb goal; the standard goal has been above 7g/dl.
This goal should be paired with a restrictive transfusion strategy to reduce the risk iron overload and alloimmunization.[4]
Platelets are typically transfused when the count is below 10,000/microL, but for septic and recurrent bleeding patients, this number should be kept above 20,000/microL.[4]
Granulocyte transfusion is usually saved for patients with both a lethal infection and neutropenia.
A Cochrane review attempted to evaluate the efficacy and safety of fibrinolytics such as tranexamic acid in patients with hematologic disorders and low platelets, but the results were inconclusive due to lack of quality evidence.[3]
Tranexamic acid is frequently used for heavy menstrual bleeding, however, even in those with inherited bleeding disorders. It has been found to reduce bleeding and improve quality of life in patients with both heavy menstrual bleeding and abnormal hemostasis.[1]
Patients with transfusion-dependent aplastic anemia should also receive prompt treatment for the aplastic anemia guided by the severity of their disease.
Severe aplastic anemia is defined as the presence of two or more of the following: absolute reticulocyte count < 60,000/microL, platelet count < 20,000/microL, and/or absolute neutrophil count < 500/microL.[6]
Very severe aplastic anemia follows the same criteria, except absolute neutrophil count must be less than 200/microL.
Moderate or non-severe aplastic anemia does not meet these criteria.
For patients younger than 40 with severe/very severe aplastic anemia, the most desired treatment is Hematopoietic Cell Transplantation (HCT).[4] Immunosuppressive therapy can be started in the interim before a suitable donor is found.
For patients older than 40 with severe/very severe aplastic anemia, triple immunosuppressive therapy with horse antithymocyte globulin, cyclosporine, and eltrombopag is recommended.
96 patients who received triple IST during a multicenter phase 3 trial demonstrated increased complete response at various time points (3 months, 6 months, and 12 months) when compared to 101 patients who only received horse antithymocyte globulin and cyclosporine alone.[5]
For those with moderate aplastic anemia, treatment is usually reserved for those with recurrent transfusions or decreased quality of life due to the pancytopenia. Experts suggest treatment with either a lower intensity IST or eltrobopag alone.[4]
RECOMMENDATIONS
The following recommendations can be made regarding adults who present with transfusion-dependent aplastic anemia:
Leukoreduced pRBCs should be transfused with a goal HgB of >7.
Platelets should be transfused in those with counts > 20,0000/microL and active bleeding.
Patients should resume/begin immunosuppressive therapy with hematology closely following.
In patients with heavy, active uterine bleeding, tranexamic acid may be added to treatment regimen.
REFERENCES
1. Dickerson, K. E., Menon, N. M., & Zia, A. (2018). Abnormal Uterine Bleeding in Young Women with Blood Disorders. Pediatric clinics of North America, 65(3), 543–560. https://doi.org/10.1016/j.pcl.2018.02.008
2. Duc N.M. & Huy H.Q. (2018). Effect of magnetic resonance imaging characteristics on uterine fibroid treatment. Reports in Medical Imaging. 11, 1-8 https://doi.org/10.2147/RMI.S162910
3. Estcourt LJ, Desborough M, Brunskill SJ, Doree C, Hopewell S, Murphy MF, Stanworth SJ. Antifibrinolytics (lysine analogues) for the prevention of bleeding in people with haematological disorders. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No.: CD009733. DOI: 10.1002/14651858.CD009733.pub3
4. Olson, T.S. & Dunbar, C.E. Treatment of aplastic anemia in adults. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on Nov. 8, 2022).
5. Peffault de Latour, R., Kulasekararaj, A., Iacobelli, S., Terwel, S. R., Cook, R., Griffin, M., Halkes, C. J. M., Recher, C., Barraco, F., Forcade, E., Vallejo, J. C., Drexler, B., Mear, J. B., Smith, A. E., Angelucci, E., Raymakers, R. A. P., de Groot, M. R., Daguindau, E., Nur, E., Barcellini, W., … Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation (2022). Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. The New England journal of medicine, 386(1), 11–23. https://doi-org.echo.louisville.edu/10.1056/NEJMoa2109965
6. Rovó, A., Tichelli, A., Dufour, C., & SAA-WP EBMT (2013). Diagnosis of acquired aplastic anemia. Bone marrow transplantation, 48(2), 162–167. https://doi.org/10.1038/bmt.2012.230
7. Sharma, R. R., & Marwaha, N. (2010). Leukoreduced blood components: Advantages and strategies for its implementation in developing countries. Asian journal of transfusion science, 4(1), 3–8. https://doi.org/10.4103/0973-6247.59384.
8. Takeuchi, M., & Matsuzaki, K. (2011). Adenomyosis: usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics : a review publication of the Radiological Society of North America, Inc, 31(1), 99–115. https://doi.org/10.1148/rg.311105110



Comments